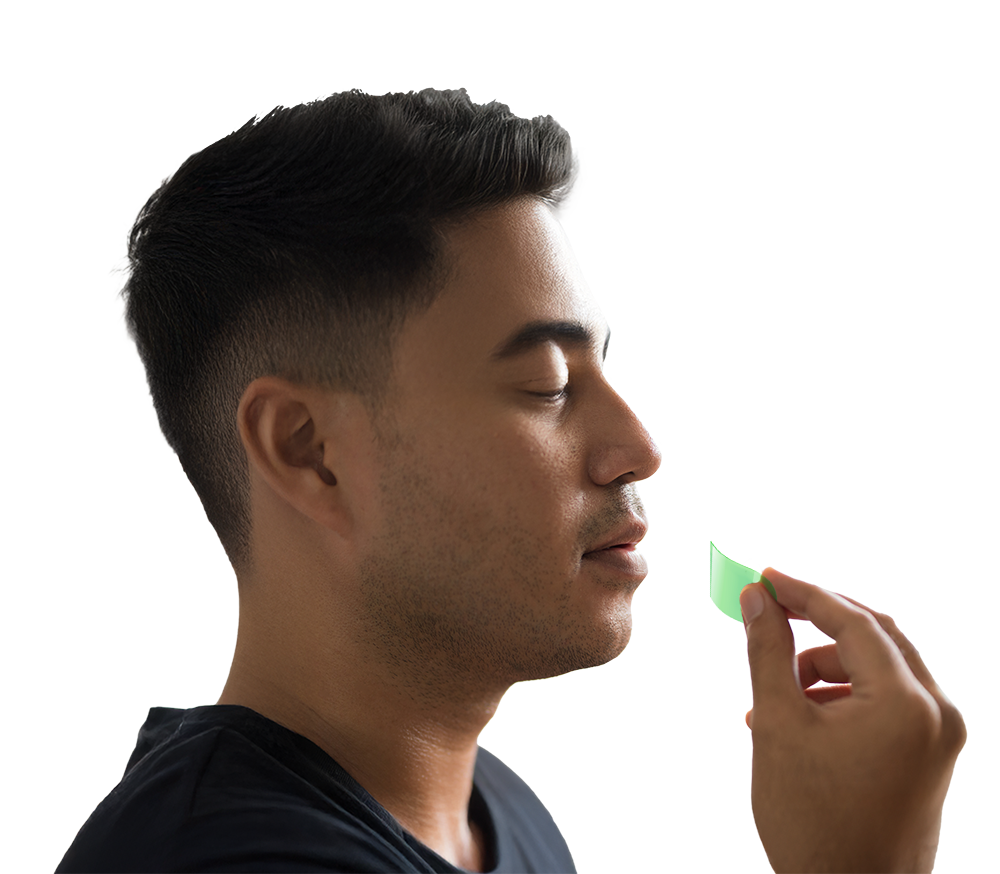
Sublingual thin films offer rapid, precise drug delivery, dissolving quickly under the tongue. Bypasses the GI tract for fast absorption and enhanced patient compliance.
Specifications
Quantifiable plasma levels as early as 5 minutes post-dose.
Bypasses first-pass metabolism.
Reduced API waste with exact dosage.
No water needed.
Dissolvable or insoluble options.
Compact, easy-to-use packaging.


Thin Film
Drug Matrix
Specifications
Sublingual thin films leverage the high vascularity of the sublingual mucosa for direct drug absorption into the bloodstream, bypassing first-pass metabolism and delivering therapeutic effects with remarkable speed.

Place the film under the tongue, where it rapidly dissolves.

The API is released and absorbed directly through the sublingual mucosa.

The drug enters the bloodstream for systemic effects, bypassing digestive processes.
Sublingual thin films deliver fast-acting, effective treatment with maximum convenience and minimal complications.


ARx offers comprehensive support throughout your development journey, from feasibility studies to full-scale production. Backed by decades of experience in polymer science and thin film technology, we ensure a reliable, high-quality sublingual film product.
Curious how sublingual thin films compare to traditional dosage forms? Explore the benefits and find the perfect solution for your unique formulation needs.
Sublingual films offer significant advantages: rapid onset (plasma levels detectable within 5 minutes), enhanced bioavailability by bypassing first-pass metabolism, precise dosing without liquid measurement, and portability resulting in improved patient compliance. This delivery system can create meaningful differentiation for your product in competitive markets.
Most small molecule APIs and even peptides with appropriate solubility and permeability characteristics are suitable for sublingual delivery. Our advanced formulation capabilities can accommodate many challenging compounds through solubility enhancement, permeation promoters, and specialized excipients. A feasibility study can determine your API’s suitability.
Our sublingual films typically dissolve within 5-30 seconds, depending on formulation requirements. This rapid dissolution ensures quick drug release for immediate absorption through the highly vascularized sublingual mucosa, making them ideal for situations requiring prompt therapeutic response.
Our sublingual films utilize pharmaceutical-grade film-forming polymers, plasticizers, disintegrants, taste-masking agents, and permeation enhancers. All excipients are USP/NF compendial or GRAS and selected specifically for their compatibility with sublingual delivery and rapid dissolution characteristics.
Our development follows a structured approach: feasibility assessment, prototype development, analytical method establishment, formulation optimization, stability studies, process development, clinical supplies manufacturer and regulatory documentation. Each phase incorporates quality-by-design principles to ensure successful commercialization.
Sublingual thin films are individually packaged in sealed pouches that protect from moisture and oxygen. Depending on formulation specifics, shelf life typically ranges from 24-36 months at ambient environmental conditions. Most formulations are stable at room temperature, making them convenient for patients and reducing distribution complexities.
Questions about our sublingual thin film options? Curious about application for your therapeutic area? Talk to our experts today about your needs.